Below are major points from the updated LCD:
- Frequency: No more than 3 TPI sessions will be reimbursed per rolling 12 months.
- Imaging guidance: The use of fluoroscopy or magnetic resonance imaging (MRI) guidance for the performance of TPI is not considered reasonable and necessary, The use of ultrasound guidance for the performance of TPI is considered investigational.
- Repeat TPI: Consistent pain relief from the most recent previous TPI lasting at least 6 weeks.
- With other Injections: It is not considered medically reasonable and necessary to perform multiple blocks (e.g., epidural steroid injection (ESI), sympathetic blocks, facet blocks, etc.) during the same session as TPI.
- Pain Scale: Documentation should be updated with the pre and post-pain scale.
——————————————-
Below are detailed updated guidelines for coverage limitations, frequency, limitation, and documentation requirements.
Coverage Guidance:
1) Initial Trigger Point Injection
Trigger point injections (TPI) will be considered medically reasonable and necessary to treat myofascial pain caused by trigger points when all the following requirements are met:
- There is a focal area of pain in the skeletal muscle;
- There is clinical evidence of a trigger point defined as pain in a skeletal muscle that is associated with at least 2 of the following findings: the presence of a hyperirritable spot and/or taut band identified by palpation and possible referred pain; AND
- The physical examination identifies a focal hypersensitive bundle or nodule of muscle fiber harder than normal consistency with or without a local twitch response and referred pain; AND
- Non-invasive conservative therapy is not successful as first-line treatment OR movement of a joint or limb is limited or blocked OR the TPI is necessary for diagnostic confirmation.
2) Subsequent TPI
Repeat TPI in previously injected trigger points will be considered medically reasonable and necessary to treat myofascial pain syndrome (MPS) when all the following requirements are met:
- There is a positive pain response from the most recent TPI defined as providing a consistent minimum of 50% relief of primary (index) pain after the TPI measured by the SAME pain scale* at baseline and post-injection; AND
- Consistent pain relief from the most recent previous TPI lasting at least 6 weeks; AND
- The myofascial pain has recurred and is causing objective functional limitations measured by a functional scale* obtained at baseline and after TPI which demonstrated at least 50% functional improvement from the previous TPI.
Limitations:
No more than 3 TPI sessions will be reimbursed per rolling 12 months
- A TPI involves the use of a local anesthetic and does not include injections of biologics (e.g., platelet-rich plasma, stem cells, amniotic fluid, etc.) and/or any other injectates.
- It is not considered medically reasonable and necessary to perform TPI into multiple muscle groups in different anatomical regions during the same session.
- It is not considered medically reasonable and necessary to perform multiple blocks (e.g., epidural steroid injection (ESI), sympathetic blocks, facet blocks, etc.) during the same session as TPI.
- TPI for treatment of headache, neck pain or low back pain in absence of actual trigger points, diffuse muscle pain, a chronic pain syndrome, lumbosacral canal stenosis, fibromyalgia, non-malignant multifocal musculoskeletal pain, complex regional pain syndrome, sexual dysfunction/pelvic pain, whiplash, neuropathic pain, and hemiplegic shoulder pain are considered investigational and therefore are not considered medically reasonable and necessary.
- Use of fluoroscopy or magnetic resonance imaging (MRI) guidance for performance of TPI is not considered reasonable and necessary.
- The use of ultrasound guidance for the performance of TPI is considered investigational.
- TPI used on a routine basis (e.g., on a regular periodic, and continuous basis) for patients with chronic non-malignant pain syndromes are not considered medically necessary.
Documentation Requirements:
- Patients must be part of an ongoing conservative treatment program and documentation to support the patient is actively participating in a rehabilitation program, home exercise program or functional restoration program is in the medical record.
- Trigger point primary index pain must be measured prior to the injection at the beginning of the session.
- The post-procedure pain level must be measured after the TPI at the conclusion of the session using the same pain scale* utilized at baseline.
- When documenting the percentage of pain relief from the primary (index) pain compared to the post-injection pain levels, it is insufficient to report only a percentage of pain relief and/or a nonspecific statement of the duration of pain relief. The documentation must include a specific assessment of the duration of relief being consistent or inconsistent with the agent used for the injection and the specific dates the measurements were obtained using the SAME pain scale* used at baseline.
- When documenting the ability to perform previously painful movements and activities of daily living (ADLs) it is insufficient to provide a vague or nonspecific statement regarding the improvement of previously painful movements and ADLs. The documentation must include a functional assessment to show clinically meaningful improvement with painful movements and ADLs, if this metric is used to justify the efficacy of the TPI procedure. Providers must use established and measurable goals and objective scales to assess functionality and ADLs measures.
——————————
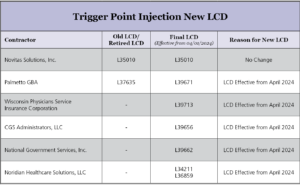
Kindly find below attachment for updated LCD for Palmetto GBA, WPS, CGS, NGS, Noradian.
